The cell and gene therapy (CGT) market is rapidly advancing, offering new hope for patients with rare diseases, cancers, and conditions that were once thought untreatable. But it remains one of the most complex areas of healthcare, especially when it comes to storing samples, ensuring stability, and maintaining long-term viability.
CGT samples are irreplaceable and must be safeguarded against degradation, loss, or noncompliance. The risks are made even more challenging by evolving global regulations and tight development timelines.
So, what’s the answer? A proactive, compliant, and globally consistent storage strategy that protects samples throughout the entire product lifecycle, from discovery and R&D to commercialization.
Sample integrity under pressure
Some CGT materials, including clinical trial patient specimens, genetically engineered intermediates, and final cell therapy products, require storage at ultra-low temperatures (-80°C) or in the vapor phase of liquid nitrogen (-135°C to -196°C) to maintain viability. These materials are particularly vulnerable to ice crystal formation, repeated freeze-thaw cycles, and microbial contamination, making temperature stability and aseptic handling critical.
Any loss or degradation of these samples can be extremely costly, or even irreplaceable, setting development programs back by months. Meanwhile, developers face the ongoing challenge of meeting tight timelines while complying with evolving and differing standards across regulatory bodies.
Evolving global scrutiny
As cell and gene therapies continue to advance, regulatory frameworks are rapidly evolving to keep pace with their growing complexity and unique risks. This includes:
- ICH Q1A(R2): Remains the foundation of stability guidance but its relevance is being interpreted more stringently for advanced therapies, particularly regarding stress conditions and shelf-life modelling.
- EU GMP Annex 1 (2022 revision): Introduces a Contamination Control Strategy (CCS) that implicates not only cleanrooms, but any storage associated with aseptic processes – relevant for CGT developers working with viral vectors or sterile components. Enforcement begins August 2025.
- FDA: Increased focus on real-time monitoring, validated storage systems, and data-driven chain-of-custody evidence.
- MHRA and EMA: Heightened scrutiny on data integrity, contamination prevention, and disaster preparedness across storage and handling.
Building resilience: a strategic approach to CGT sample stability and biorepository storage
To mitigate risk, meet rising regulatory expectations and achieve cost efficiencies, CGT innovators need an integrated stability and biorepository storage partner. They need infrastructure, validation expertise, and inspection-readiness built into every layer of operations. An ideal partner offers:
- Comprehensive breadth of sample stability and storage temperatures and environmental control capabilities.
- Proven, compliant infrastructure validated for conditions ranging from +4°C short-term refrigeration to long-term cryogenic preservation (−196°C).
- GMP-qualified cryobags or vials, cryoprotectants like DMSO, and controlled-rate freezing systems for preserving cell viability.
- Disaster recovery and continuity systems designed to protect high-value materials from outages, excursions, or environmental failures.
- Validation expertise to support inspection readiness, equipment qualification, and audit trail assurance.
- A global footprint and deep understanding of regional regulatory nuances, especially as trials scale internationally.
- Scalable capacity to grow with a program from early-phase research to commercial supply.
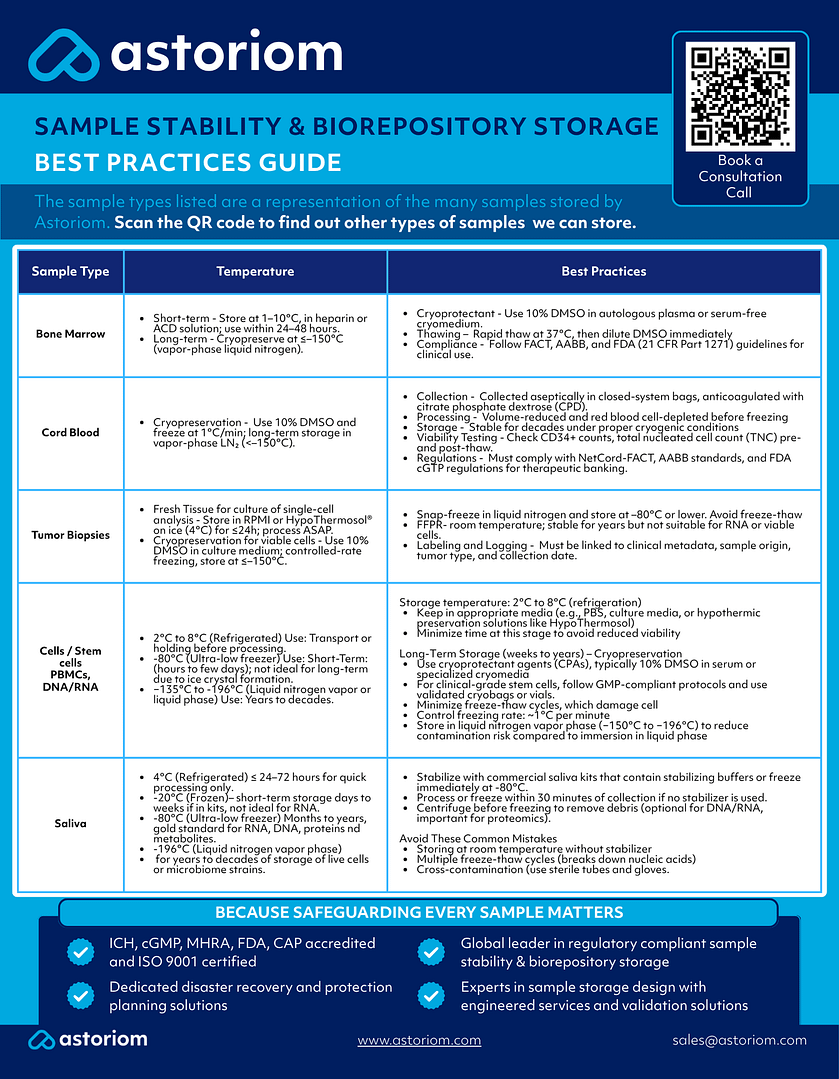
Best practices for CGT sample storage and stability
Safeguarding CGT samples requires more than compliant facilities. It demands rigorous, scientifically validated practices that account for the fragile nature of these high-value materials. These are the core principles that underpin safe, stable, and inspection-ready storage:
Use of cryoprotectants
Use agents like DMSO (typically 10%) for viable cell storage. Combine with appropriate media (e.g., HypoThermosol) to enhance post-thaw recovery.
Tip: Always control freezing rate (~1°C/min) and use a validated cryogenic storage method (e.g., vapor-phase liquid nitrogen). For clinical-grade materials, post-thaw washing to remove DMSO is typically required to reduce toxicity.
Minimize freeze-thaw cycles
Aliquot samples to avoid repeated freeze-thaws, which degrade nucleic acids, proteins, and cell viability.
Maintain ultra-low temperatures
Some CGT materials require:
- −80°C for DNA, RNA, plasma, proteins.
- −135°C to −196°C for cells and live biologics.
Avoid frost-free freezers and ensure continuous temperature monitoring with alarms.
Control contamination
Follow GMP principles in aseptic sample handling. Use sterile containers, validated cleanroom protocols, and contamination control strategies as required by EU GMP Annex 1.
Ensure chain of custody and traceability
Each transfer point—from site to central biorepository—must be logged digitally. Include metadata: sample ID, storage conditions, excursions, corrective actions.
Validate all equipment and SOPs
Freezers, incubators, and monitoring systems must be qualified and maintained under documented calibration and performance checks.
Astoriom’s approach: complete confidence, end-to-end
At Astoriom, we believe sample storage shouldn’t be an afterthought. With over 30 years of experience supporting pharmaceutical, biotech, and advanced therapy developers, our goal is to help companies protect their CGT materials with confidence—no matter where they are in the product lifecycle.
We operate facilities in key locations, including both the East and West Coasts of the United States, so teams can count on local support for US-based and global studies. We also have locations in the UK and Ireland that support organizations with European operations. Our services cover everything from discovery-phase storage and stability testing to GMP-qualified equipment, validation, and robust disaster recovery planning.
Regulatory alignment is built into everything we do, meeting ICH, CAP, cGMP, FDA, EMA, HTA, and local standards—so that every SOP, monitoring system, and record stands up to inspection. Digital audit trails and detailed excursion logs mean teams can stay focused on advancing therapies, knowing their samples are protected and fully traceable.
For us, secure storage isn’t just about temperature and freezers—it’s about helping innovators build trust in the safety, integrity, and compliance of their programs, wherever their work takes them next.
What’s new: emerging regulations and expectations
As the cell and gene therapy landscape evolves, so too do the rules that govern how sensitive materials must be handled and protected. New guidance and updates from global regulators are setting higher expectations across several critical areas:
Chain of custody: Updated USP <1079.2> and recent FDA guidance now call for documented control at every transfer point—not just within your own facilities, but across third-party vendors and digital systems too.
Contamination control: The revised EU GMP Annex 1 makes it clear that contamination prevention doesn’t stop at the cleanroom door. Storage zones connected to aseptic processes must meet the same rigorous standards.
Disaster preparedness: Having an SOP on paper isn’t enough anymore. Regulators want proof that emergency procedures are tested, redundant power systems are in place, and real-time risk monitoring is active.
Data integrity: With more focus from the FDA, MHRA, and EMA, validated and secure data systems for monitoring and logging storage conditions are essential. Manual records or unverified systems won’t pass muster.
Holistic quality oversight: From temperature monitoring and equipment calibration to excursion management and recordkeeping, end-to-end quality systems are now the baseline for inspection readiness.
Staying ahead of these updates requires a proactive approach and trusted partners who can help keep your operations resilient as expectations continue to rise.
Storage is strategic, not secondary
It’s clear that for CGT companies to succeed, sample storage must feature as a strategic pillar of successful drug development. With the right partner, CGT innovators can navigate complexity, mitigate risk, and scale confidently. Astoriom is proud to support companies from discovery through to delivery, with resilience, compliance, and partnership at every stage.
Ready to strengthen your storage and stability strategy? Explore how Astoriom can support your advanced therapy programs with confidence; get in touch with us today
About Astoriom
At Astoriom, we are the global quality expert in safeguarding R&D sample assets.
For over 30 years, our customers have trusted us to secure, protect and preserve their scientific and consumer R&D product samples. We offer a comprehensive portfolio of sample stability storage, biorepository storage, disaster protection & recovery, and sample storage equipment & validation services. Our global environmentally controlled storage solutions enable our customers to safeguard the integrity and viability of their valuable sample assets to advance their research innovation. As a global leader and trusted expert in sample stability and biorepository storage solutions, our team of specialists and engineers offer international regulatory compliant quality processes and custom designed sample storage protocols to the top R&D industries worldwide.




